Abstract
Introduction Waldenström macroglobulinemia (WM) is a rare lymphoplasmacytic lymphoma that can manifest with impairment of hemostasis and platelet dysfunction. Very rarely, patients (pts) with WM can develop acquired von Willebrand syndrome (AVWS), a bleeding disorder generally associated with another systemic process. There is limited literature demonstrating the association of these conditions, and the clinical manifestations and symptomatology associated with treatment remain poorly explored. Our study aimed to assess the prevalence of this condition at our institution and determine the associated clinical manifestations and outcomes.
Methodology Consecutive pts with a diagnosis of WM evaluated at Mayo Clinic, MN, FL and AZ who underwent von Willebrand factor (VWF) testing from 01/2002 to 01/2022 were included. Laboratory confirmation was obtained in the Special Coagulation Laboratory at Mayo Clinic, and those with a confirmed diagnosis of WM and AVWS were included in our final cohort. Bleeding symptoms, WM- and AVWS-related treatment, and associated laboratory data were abstracted.
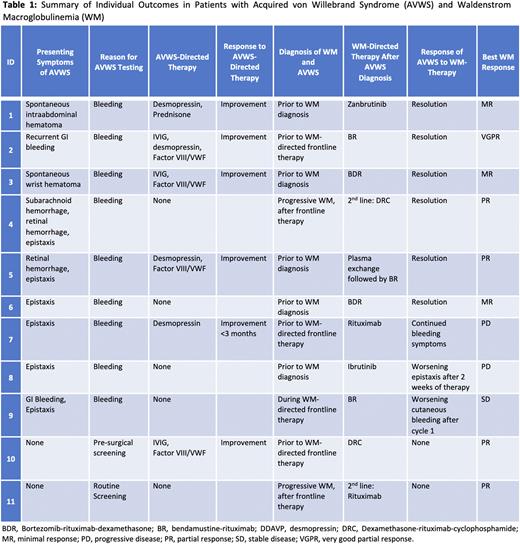
Results Of 2210 pts with a diagnosis of WM, 73 (3%) received testing for VWF, and 11 (15% of those tested and 0.5% of all pts) were diagnosed with AVWS. The median follow-up for these 11 pts was 34.0 months (95% CI, 30.1 - 163.6). Baseline testing at time of initial AVWS diagnosis [median (range, normal)] revealed: platelet count 170 (49-281, 135-317), VWF-activity (latex immunoassay) 25% (12-31, 55-200), ristocetin co-factor activity 21% (12-31, 55-200), VWF-antigen 32 (13-44, 55-200), and Factor VIII activity 40% (16-64, 55-200). Multimer analysis available in 6 patients revealed a normal distribution pattern.
The most common underlying reason for testing was bleeding symptoms, observed in 9 (82%) pts. Other reasons for testing included pre-surgical (n=1) and routine screening (n=1). Eight (73%) pts were diagnosed with AVWS prior to frontline treatment initiation for WM. Concurrent diagnosis of AVWS and WM was made in 5 (45%) pts, 3 (27%) pts were diagnosed with AVWS before the progression of smoldering WM to WM and WM-directed therapy initiation, 2 (18%) pts were diagnosed during progressive WM after frontline therapy, and 1 (9%) pt was diagnosed during frontline therapy. In the 5 pts with concurrent diagnosis, WM was diagnosed a median of 35 days (range, 4-83) after the diagnosis of AVWS. All pts received WM-directed treatment.
Recurrent epistaxis was the most observed bleeding symptom and was seen in 6 (55%) pts. Other observed symptoms included spontaneous hematoma (n=2), gastrointestinal (GI) bleeding (n=2), retinal hemorrhage (n=2), and subarachnoid hemorrhage (n=1). Seven (64%) pts had a major bleeding event leading to hospitalization due to spontaneous hematoma (n=2), GI bleeding (n=2), epistaxis (n=2) and subarachnoid hemorrhage (n=1). Two (18%) pts required surgical embolization due to severe epistaxis. Pharmacologic hemostatic treatments were administered in 6 (55%) pts, including Factor VIII/VWF complex (n=4, 36%), administered due to GI bleed, hematoma, epistaxis, and pre-surgical prophylaxis. Desmopressin was administered to 4 (36%) pts for hematoma, GI bleed, and epistaxis. Three (27%) pts received intravenous immunoglobulin (IVIG) for GI bleed, hematoma and pre-surgical prophylaxis, and 1 (9%) pt received prednisone for hematoma. These therapies led to at least temporary bleeding symptom improvement (<3 months) in all 6 pts. Of the 9 pts who had bleeding symptoms, 6 (67%) had improvement in their symptoms after WM-directed therapy. Three pts who were refractory to WM-directed therapy had no improvement in bleeding symptoms while on WM therapy (Table).
Conclusions We found that <1% of pts had an identified diagnosis of AVWS secondary to WM, highlighting the rarity of this diagnosis and its potential under-recognition. The bleeding risk associated with AVWS in combination with WM, a malignancy inherently associated with bleeding, likely underlies the high rates of life-threatening bleeding events. The high morbidity associated, leading to events such as CNS bleeding and retinal hemorrhage, signals that one needs to evaluate pts with WM who are presenting with symptomatic bleeding for AVWS. Additionally, given a fraction of pts had worsening bleeding events after therapy initiation, agents with lower bleeding risk should be considered in pts with this diagnosis.
Disclosures
Witzig:Karyopharm: Other: Clinical Trail Support; ADC Therapeutics: Membership on an entity's Board of Directors or advisory committees; Curio Science: Honoraria; Kura Oncology: Other: Clinical Trail Support. Ansell:SeaGen: Research Funding; Takeda: Research Funding; Bristol Myers Squibb: Research Funding; Regeneron: Research Funding; Affimed: Research Funding; Pfizer: Research Funding; ADC Therapeutics: Research Funding. Kumar:AbbVie,: Membership on an entity's Board of Directors or advisory committees, Research Funding; Celgene: Membership on an entity's Board of Directors or advisory committees, Research Funding; Janssen,: Membership on an entity's Board of Directors or advisory committees, Research Funding; Takeda,: Membership on an entity's Board of Directors or advisory committees, Research Funding; Adaptive,: Membership on an entity's Board of Directors or advisory committees, Research Funding; KITE,: Research Funding; MedImmune/Astra Zeneca,: Membership on an entity's Board of Directors or advisory committees, Research Funding; Merck,: Research Funding; Novartis,: Research Funding; Roche: Research Funding; Sanofi: Research Funding; Oncopeptides: Other: Independent review committee. Pruthi:Instrumentation Laboratory: Honoraria, Membership on an entity's Board of Directors or advisory committees; HEMA Biologics: Honoraria, Membership on an entity's Board of Directors or advisory committees; Bayer Healthcare AG: Honoraria, Membership on an entity's Board of Directors or advisory committees; Genentech Inc: Honoraria, Membership on an entity's Board of Directors or advisory committees; CSL Behring: Honoraria, Membership on an entity's Board of Directors or advisory committees; Merck: Consultancy. Kapoor:GSK: Honoraria; Imedex: Honoraria; Pharmacyclics: Honoraria; Casma: Honoraria; Takeda: Research Funding; AbbVie: Honoraria, Research Funding; BMS: Honoraria, Research Funding; Karyopharma: Research Funding; Loxo: Research Funding; Ichnos: Research Funding; Amgen: Research Funding; Regeneron: Research Funding; Sanofi: Honoraria, Research Funding; Cellectar: Honoraria; Oncopeptides: Honoraria; X4 Pharmaceuticals: Honoraria. Sridharan:Alexion Pharmaceuticals: Consultancy.
Author notes
Asterisk with author names denotes non-ASH members.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal